Dr. Bonnie Henry says she's 'excited' about news of success with experimental drug for COVID-19
U.S. study suggests antiviral drug remdesivir reduced average recovery time
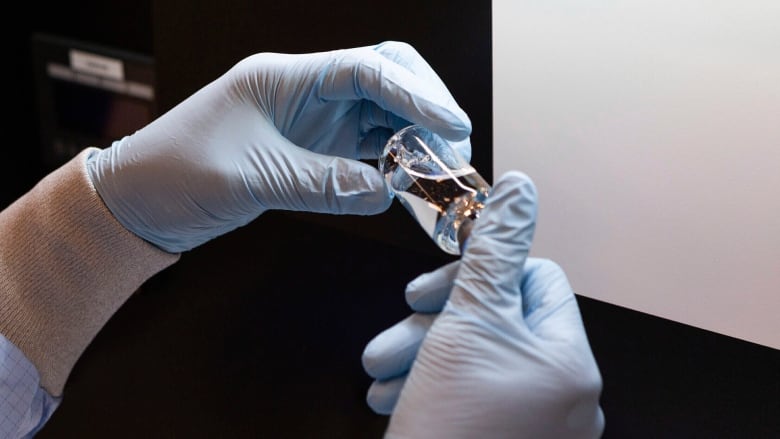
B.C.'s Provincial Health Officer says she's cautiously optimistic about a new study on the experimental antiviral drug remdesivir's effects on COVID-19.
American officials revealed this week that research conducted by the U.S. National Institutes of Health found remdesivir reduced recovery time by 31 per cent in COVID-19 patients — from 15 to 11 days on average.
During her daily briefing on Thursday, Dr. Bonnie Henry described those results as good news.
"I think it's a very positive thing, but with cautious optimism, because we don't yet know all the details," Henry said.
Remdesivir is an antiviral drug, which means it doesn't prevent infection, but it makes it more difficult for the virus to reproduce and spread through the body. It's manufactured by Gilead Sciences Inc., and the U.S. Food and Drug Administration is now reportedly working to make the drug quickly available to patients.
'It looks promising'
The American results come from a randomized, controlled and blind trial of 1,063 hospitalized coronavirus patients.
"It appears that for people who have severe illness — they're hospitalized or in ICU — it might be an effective drug to help them recover quicker," Henry said.
"In that context, it looks promising and that's very good news, and it's something we should all be excited about."
But she said scientists in B.C. haven't had a chance to see the data from the study. There are also Canadian trials looking at remdesivir's effectiveness, along with that of other potential treatments.
"We need to continue the randomized controlled trials to have a better understanding of exactly how it works, how much, and who it's going to work best for," Henry said.